Research
Development of innovative solid CO2 absorbents
Background and need for research
Atmospheric CO2 concentrations are increasing at an accelerating rate (NOAA, USA), casting doubt on the viability of the human race. According to the IEA's roadmap published in 2021, global CO2 emissions should have been slowing down by 2025 and reduced to around 30 billion tons per year (p. 152 of the roadmap), but in reality, it has been reported that the global emissions have exceeded the 40 billion ton (40 gigatons) mark in 2023 (Energy Institute, UK, 2024). It has been scientifically proven that the increase in atmospheric CO2 concentration contributes to global warming (Syukuro Manabe, Nobel Prize in Physics 2021) and that the increase in atmospheric CO2 concentration is primarily anthropogenic (IPCC AR6 WG1, Chapters 1 & 3; see also the website of the Royal Society). In fact, the global temperature has been steadily increasing (Copernicus, EU), and in particular from 2023 to 2024, it clearly exceeded the previous global average temperature and set a new record high (Copernicus, EU).
The recent climate changes are causing natural disasters (UNEP), hungers (WFP), and health problems (WHO) on a global scale. From a different perspective, some researchers pointed out fundamental problems with humans as a species, such as the concern that humans may not be able to maintain concentration (and hence intellectual activities may be hindered) if CO2 concentrations exceed 1000 ppm (Satish et al., 2012). In this way, the problem of rising atmospheric concentrations of CO2 not only jeopardizes the future survival of humanity but also poses an imminent danger to our current lives and the order and well-being of our society.
When considering solutions to this problem, the most important perspective is the "quantity perspective." As mentioned above, humans currently emit approximately 40 billion tons of CO2 annually. Japan emits about 1 billion tons annually (Ministry of the Environment of Japan, April 2022). In particular, Japan's top 10 emission sources emit more than 10 million tons at a single location (Kiko Network, 2017, in Japanese).
In recent years, research on 'carbon capture and utilization (CCU)' has become active. While CCU has a certain significance as a way to respond to economic and social requirements to reduce emissions through carbon pricing or an ESG viewpoint, the following three perspectives are considered important. (1) Is the amount of its reuse meaningful compared to the CO2 emissions of the above order? (2) How does the CO2 used for reuse come to be separated? (3) Energy input is required for CO2 separation and reactions such as CO2 reduction (and in some cases, the generation of hydrogen to be reacted with CO2), but are these energies available? In other words, if the context is to solve the climate change problem, a quantitative and systematic perspective is needed.
A representative technology for a quantitative solution is the geological sequestration of CO2, known as 'carbon capture and storage (CCS),' which is already in large-scale operation around the world (Global CCS Institute). However, the more we envision the capture of CO2 from large-scale emission sources, the more we are faced with the question of how to do it and whether the energy and equipment costs for capture are realistic. The current technology is the aqueous amine solution method described next; however, this has problems, and therefore, research is needed to solve the problems.
Problems with existing technology and points that require improvement
The current technology is a chemical absorption method that uses an aqueous solution of amine molecules (e.g., primary amines are -NH2), in which CO2 molecules react with the amine molecules in the solution and are captured (Renew. Sustain. Energy Rev., 2022). When the CO2 is desorbed (or the solution is regenerated), thermal energy must be applied to break the chemical bonds formed between the CO2 and the amine molecules. However, the primary problem with the current technology is that it is also necessary to heat the solvent water, which makes up most of the weight of the liquid (sensible heat), and to input the additional large amount of thermal energy to turn the water into vapor (latent heat). Although improvements in absorbent solutions are currently being made (COURSE50, in Japanese), they are mainly based on efforts to reduce the heat of reaction between amine molecules and CO2 molecules, not on reducing the sensible and latent heat of water as a solvent. There is a limit to the approach of lowering the heat of reaction, because the reaction rate and heat of reaction are inversely related (COURSE50, in Japanese). Therefore, lowering the heat of reaction too much will result in an economically unacceptable equipment size due to the need to increase the contact time between the amine solution and flue gas, since liquid is generally a slow diffusion medium for gas molecules.
In addition, aqueous solutions of amines are highly corrosive to metals and harmful to the ecosystem and human health (Renew. Sustain. Energy Rev., 2022), which increases, respectively, the cost for frequent maintenance inspections of equipment and replacement of amine solutions and the cost for scrubber facilities to prevent the release of solute amine molecules into the environment. In other words, if we are aiming to lower the heat of reaction, dramatically reduce the energy input required in the regeneration process, and solve environmental problems, we need to stop using aqueous solutions.
Therefore, the necessary improvements are (1) to avoid using water, which causes excess heat capacity (sensible & latent heat) and low gas diffusion coefficient, i.e., to make it solid, (2) to ensure high reaction rate by using porous solids rather than tight solids, and (3) not to use support materials that can cause excess heat capacity and weight. Among them, (2) is necessary to avoid the above-mentioned dilemma that reaction rate and reaction heat are in a trade-off relationship. If gas diffusion and reaction are faster in porous media, the heat of reaction, which is a part of the energy input required in the regeneration process, can be reduced without side effects.
Idea and aim
The idea of this research is to use "covalent organic frameworks (COFs)" explained below, as a porous material that can effectively solve the above-mentioned problems. COFs are crystalline organic porous materials that have attracted much attention recently because they are composed only of light elements (usually C, H, O, and N), metal-free, highly durable because they are constructed with covalent bonds, and have nanoscale pores that are sufficiently larger than CO2 molecules. COFs are, to say, "jungle gyms" consisting of molecular rods, and their pore size and function can be designed by selecting building block molecules. We believe that using COF, specifically if amine adsorption sites can be densely arranged on the COF's skeleton, we can create the ultimate solid CO2 absorbents that are solvent-free, support-free, porous, highly stable, and consist of only light elements.
Because COFs are organic solids, they must be kept below 200°C for long-term use in the presence of oxygen. This is a much lower temperature than the temperatures used in the methods that use tight-lattice inorganic materials; this is because the gas diffusion coefficient (or mass transfer coefficient) of tight-lattice inorganic materials is small, and hence such inorganic materials require the use of high temperatures to ensure the reaction rate.
Furthermore, the use of relatively low temperatures has principled advantages based on thermodynamics. Simply put, the act of separating gases to lift off the mixing state at a high temperature will, in principle, require higher energy input for the gas separation, because a high temperature favors disorder or the state with increased entropy. If one wants to reduce the energy input for CO2 separation, separation and recovery should be performed at as low temperatures as possible, which is rooted in the fundamental principles of science. (Note: There is a limit because too low temperatures slow down the rate of molecular diffusion and desorption within the material, but if there is a "porous material with low heat of adsorption," that will be avoided. If a tight-solid or liquid is used, however, the mass transfer coefficient will be lowered, so higher temperatures are needed to ensure the reaction rate.)
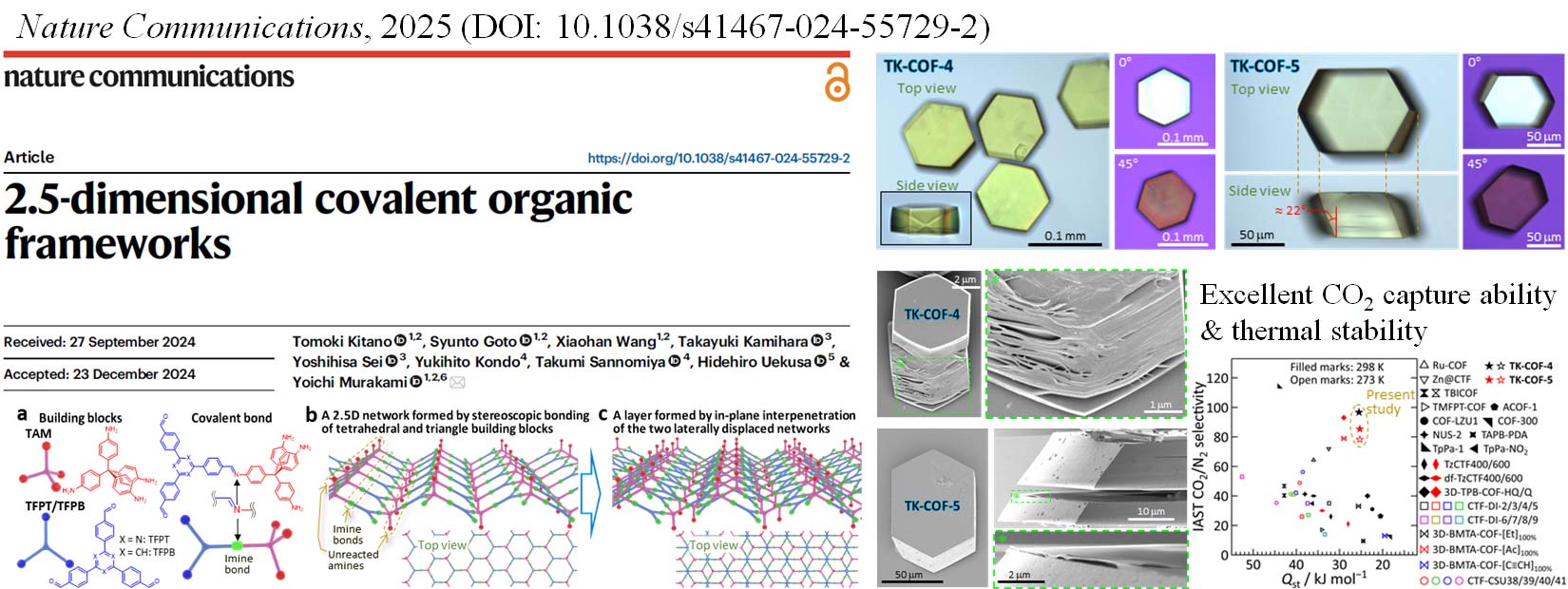
We are conducting such research from the development of COFs to the system-level performance evaluations, to create an innovative CO2 capture and separation technology that can be deployed on a large scale to mitigate climate change. We recently published a paper reporting a new class of COFs with excellent CO2 capture properties. We are currently aiming to achieve even higher performance and lower costs to realize industrialization.
Related news release: "New 2.5-dimensional skeletons in porous organic crystals are key to superior CO2 separation," EurekAlert! (AAAS)
Development of innovative solid electrolytes for solid-state batteries
Background and need for research
The rapid increase in variable renewable energy sources due to the recent spread of solar and wind power generation has led to frequent output curtailment (disconnecting renewable energy sources from the grid because they cannot be consumed by the demand side), which was previously unthinkable. This has not only led to the waste of renewable energy but also caused large price fluctuations in the electricity trading market. In the United States, Europe, and Australia, the expansion of renewable energy has even led to negative electricity market prices (i.e., people paying a fee to have the electricity they generate taken away). An effective solution to this problem is to store electricity at the generation site using secondary batteries and adjust supply and demand by shifting it over time.
Furthermore, in recent years, there has been a trend toward the electrification of vehicles and other mobile objects, with the aim of reducing CO2 emissions, promoting the use of renewable energy, and combating environmental pollution in residential areas. Drones are also a type of mobile object. For such purposes, secondary batteries are essentially required to be lightweight and have a high energy density to maximize driving range, and to be highly stable against various external disturbances to maximize safety.
However, most current secondary batteries use organic solvents as electrolytes, which increases the risk of fire. This is because organic solvents generally have a boiling point of around 80 to 200 °C, vaporize easily, and become flammable gases when mixed with air. For this reason, serious fire accidents have occasionally occurred in portable electronic devices (Forbes, 2023), power storage facilities (MIT Technology Review, 2025; PV Magazine, 2023), automobiles (EV FireSafe; MIT Technology Review, 2025), and aircraft (2013 Boeing 787 Dreamliner grounding, Wikipedia).
One promising solution to this problem is all-solid-state secondary batteries, which eliminate the boiling point, vapor pressure, and the possibility of forming flammable gases, by solidifying the electrolyte. This technology field is the subject of intense R&D competition. However, as described below, even current all-solid-state batteries have problems. The one background to the problems is that most R&D efforts to date have been focused on 'inorganic' systems, where material exploration is limited to the scope of the periodic table, while limited R&D efforts have been made on 'organic systems,' which have virtually infinite design freedom. We believe that using less-explored organic materials, when combined with the ideas described below, will be effective in solving the problems of current inorganic all-solid-state batteries and achieving lightweight and high-performance. This is why research on this topic is necessary.
Problems with existing technology and points that require improvement
The materials currently used for the solid electrolytes of all-solid-state secondary batteries (especially all-solid-state lithium-ion secondary batteries) are inorganic, and can be roughly classified into 'sulfide-based' and 'oxide-based' materials (Adv. Energy Mater., 2021). The common problem is that the electrolyte is an inorganic solid with a low degree of deformation freedom. As the active material repeatedly expands and contracts during charging and discharging, the interfaces between the electrolyte, electrodes, and active material (which should be in close contact) tend to peel off, resulting in a loss of contact between them (Chem. Rev., 2020). Because sulfide-based materials are softer than oxide-based materials, interfacial delamination can be reduced by applying pressure to restrain the expansion of the cell; however, the use of such a constraining container leads to an increase in the battery weight as well as the cost.
Currently, the types of batteries being considered for use in vehicles, which require large capacity, are sulfide-based solid electrolytes, which are relatively flexible (compared to oxide-based ones) and have high ionic conductivity. Japan is leading the way in this technology field. For example, Idemitsu Kosan and Toyota Motor Corporation are collaborating to achieve mass production (News release from Toyota, 2023). This sulfide-based solid electrolyte is made from sulfur components generated during the manufacturing process of petroleum products, and has the potential for large-scale production. Because it is sulfide-based, it is said to be crack-resistant (Toyota Times, 2023). However, sulfide-based solid electrolytes have the drawback of generating highly toxic hydrogen sulfide (H2S) gas when they come into contact with moisture in the air, even at very low moisture concentrations (ACS Appl. Energy Mater., 2024). Additionally, strict dehumidification control is required during the manufacturing process, which significantly increases manufacturing cost (example: descriptions in the webpage of atomfair.com).
Oxide-based solid electrolytes, which can avoid this drawback, have been put to practical use in small, low-capacity batteries. However, they generally have low ionic conductivity around room temperature, and the material particles are hard and brittle, so they need a hot-press sintering at high temperature for several hours (Small, 2023). In addition, their brittleness makes them poorly compliant with volume expansion and contraction. Furthermore, some applications, such as drones, require not only safety but also light weight, making it important to reduce the weight of the restraining container used to prevent the aforementioned interfacial peeling.
One way to achieve significant improvement would be to soften the solid electrolyte so that it could follow the volume change of the electrode active material, but inorganic materials are usually hard and brittle; this is the root of the difficulty. The flexibility issue could be resolved by switching to organic materials such as polymers and plastics. However, existing solid-state lithium polymer batteries have a drawback in that the dense polymer electrolyte has low ionic conductivity, resulting in a generally low transference number (the fraction of current carried by lithium ions in the total current; in inorganic electrolytes, it is often close to 1).
Idea and aim
To solve the above-mentioned problems, this research aims to develop a new solid electrolyte that satisfies the following three points: (1) It is an organic material that is flexible and allows for a high degree of freedom in material design. (2) The material is not dense, but rather a crystalline porous material that can achieve high ion conductivity (at least with micropores larger than the conducting ions). (3) The material can conduct only positive and negative ions (i.e., single-ion conductive) and hence achieve a transference number of 1.
As a material system that can satisfy these requirements, we have chosen "covalent organic frameworks (COFs)" explained below. COFs are crystalline porous polymers and are a class of materials that have been attracting attention in recent years, because their structure and properties can be designed with a high degree of freedom. The above condition (3) can be achieved by using building block molecules that ionize the framework, thereby imparting selectivity to the COF for ions of the opposite charge. Although the synthesis of such COFs has been reported in several previous studies, their crystallinity was significantly low, and they appeared amorphous even when viewed under a scanning electron microscope (example 1: page 22 of SI of ACS Energy Lett, 2020; example 2: page 8 of SI of Nano Lett, 2021). Low crystallinity poses the following obstacles to achieving the above-mentioned objective (application as an electrolyte in solid-state batteries). The channels or pores of a COF with single-ion conductivity can be compared to a highway. However, if the highway ended every 100 meters with a toll booth, followed by a short, slow-speed road, followed by another 100-meter section of highway, and so on, the benefits of the highway would not be fully utilized. Each section of highway would need to be at least tens of kilometers long. In this analogy, the length of the highway section corresponds to the grain size of the individual COF crystallites that make up the material. Increasing crystallinity increases the grain size, which allows ions to transport more smoothly, i.e., improves ionic conductivity.
The reason for the low crystallinity in previous research is that COFs are constructed using highly stable (high binding energy) covalent bonds (in the field of COFs, this is called the crystallization problem). On the other hand, through our efforts to date, we have accumulated know-how for producing COFs with high crystallinity. In particular, we believe that our technology for generating highly crystalline COFs and for structural analysis is at a very high level. Furthermore, the COF skeleton can often deform like a pantograph, making it highly adaptable to large deformations (example: page 27 of SI of our paper, JACS, 2024). Furthermore, low-density COFs (less than about 0.5 g/cm3) can also have the mechanical flexibility of a sponge (example: page 27 of SI of our paper, JACS, 2025).
COFs that satisfy the above (1) to (3) should be possible by selecting the building block molecules properly. However, the reason this has not yet been achieved is that COFs have not yet been produced with high crystallinity. We believe that if COFs with crystal sizes of around tens of microns can be produced, it will be possible to form the "highway with a section tens of kilometers long" mentioned above. Furthermore, as a more challenging attempt, we are creating membranes of COFs. If this is achieved well, it is expected to become a game-changing, innovative material that will solve the problems of the current inorganic-based electrolytes.
(Note: The COFs need to be resistant to oxidation and reduction to prevent themselves from decomposing, and this may be achieved by increasing the oxidation state and reduction state of the COFs, respectively. One of the features of COFs is that, because they are porous, the entire framework can be oxidized or reduced by post-treatment after COF formation.)
About covalent organic frameworks (COFs)
Covalent organic frameworks (COFs) are an emerging class of materials, first reported in 2005 (2D-COFs) and 2007 (3D-COFs). COFs are crystalline porous solids formed by regularly and periodically polycondensing building block molecules. Because no substance is finer than a molecule, COFs are the finest 'jungle gym' that can be created. The function and pore size can be designed by choosing building block molecules. Since COFs are usually composed of light elements only, they are metal-free, lightweight, easy to dispose of, and environmentally friendly. Covalent bonds contribute to the high thermal and chemical stability of COFs. Because of these advantages, COFs have been proposed for a variety of applications.
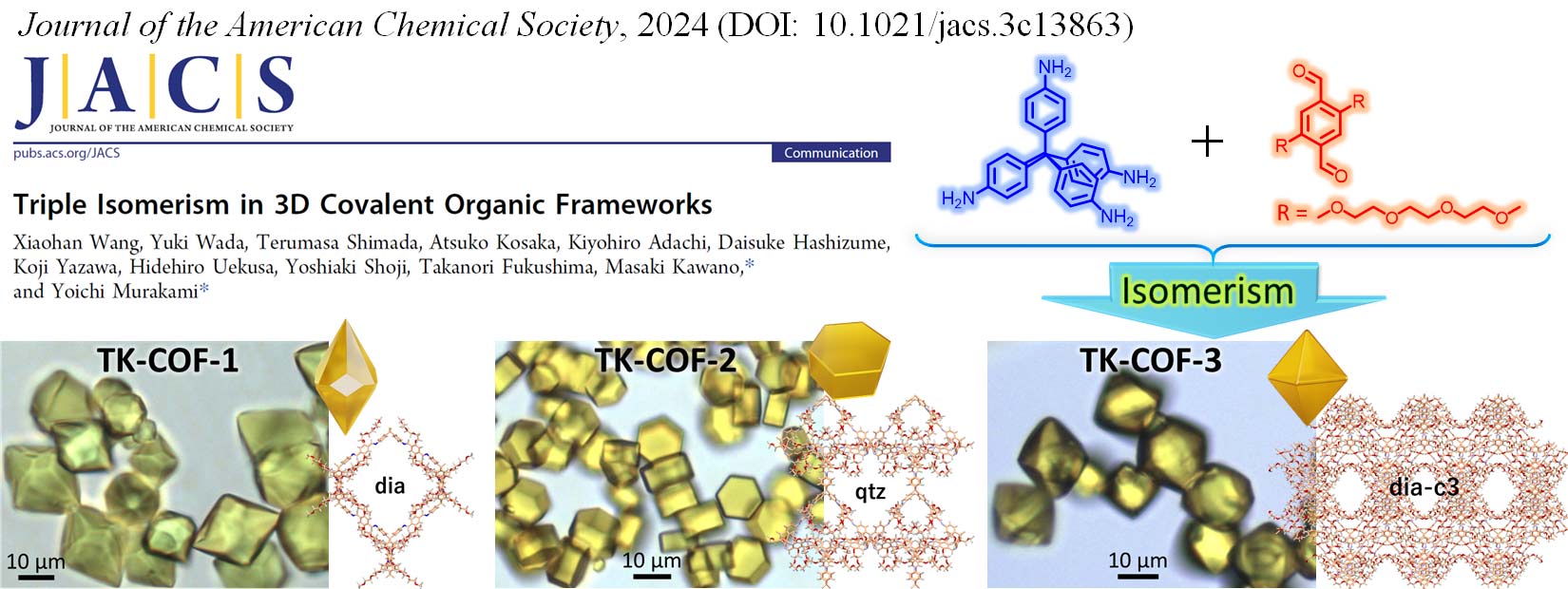
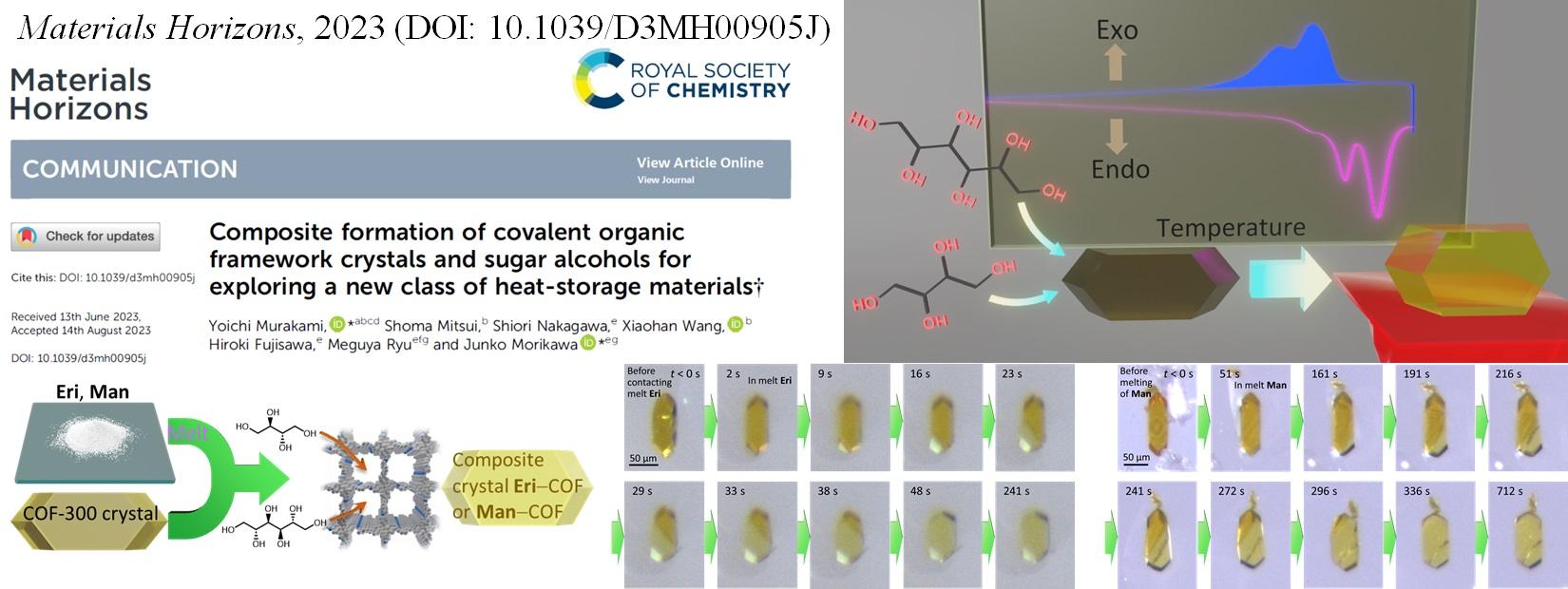
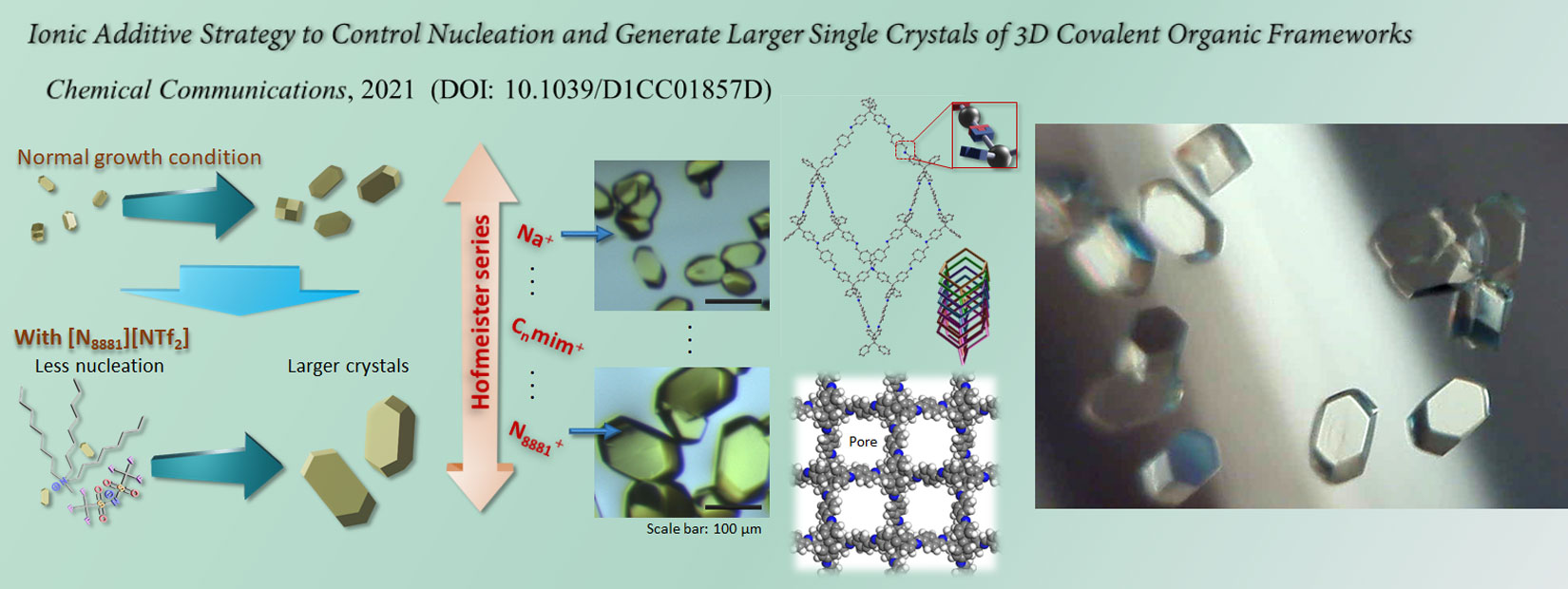
We began researching COFs in 2017. We have so far achieved the growth of high-quality, large-size single crystals through nucleation control strategy (Chem. Commun. 2021), the creation of composite heat storage materials (Mater. Horiz. 2023, news release), the diversification of framework structures through the generation of structural isomers (JACS 2024, news release), the creation of COFs with new structures and outstanding CO2 separation and capture performance (Nature Commun. 2025, news release), and the creation of electric-field-responsive COFs with new structures and dipolar rotors (JACS 2025, news release).
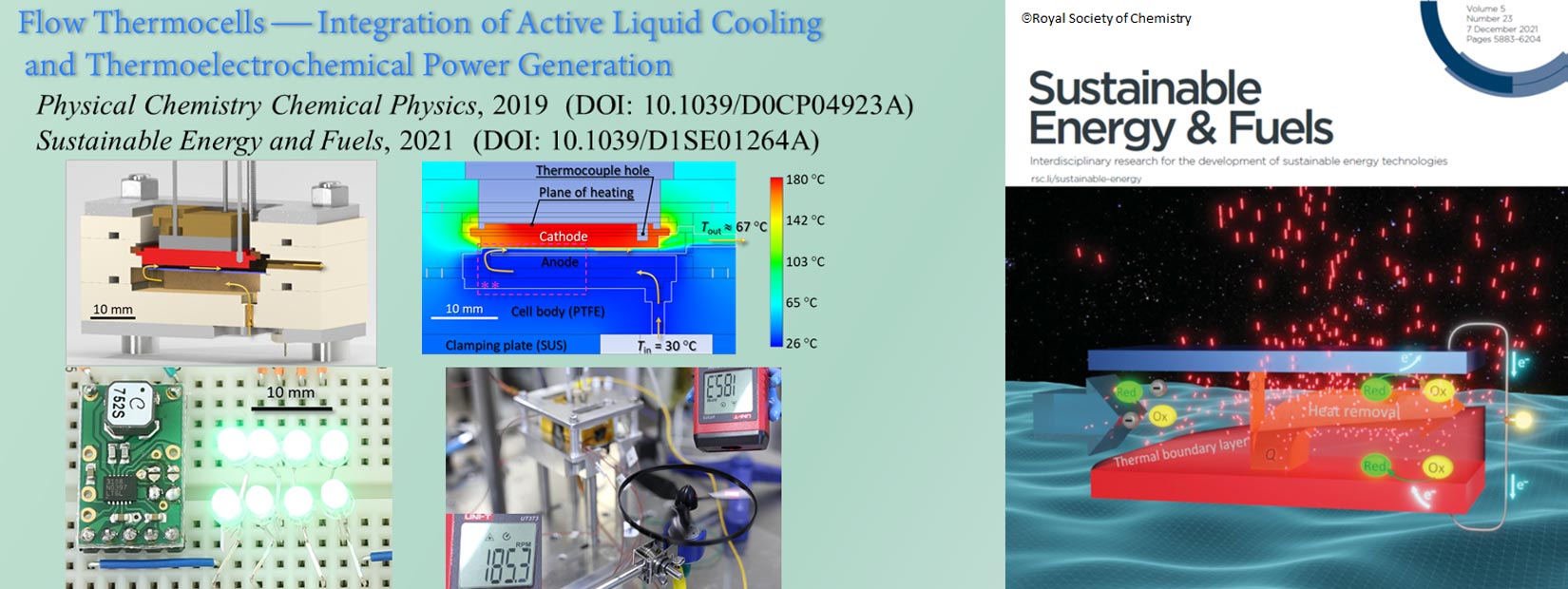
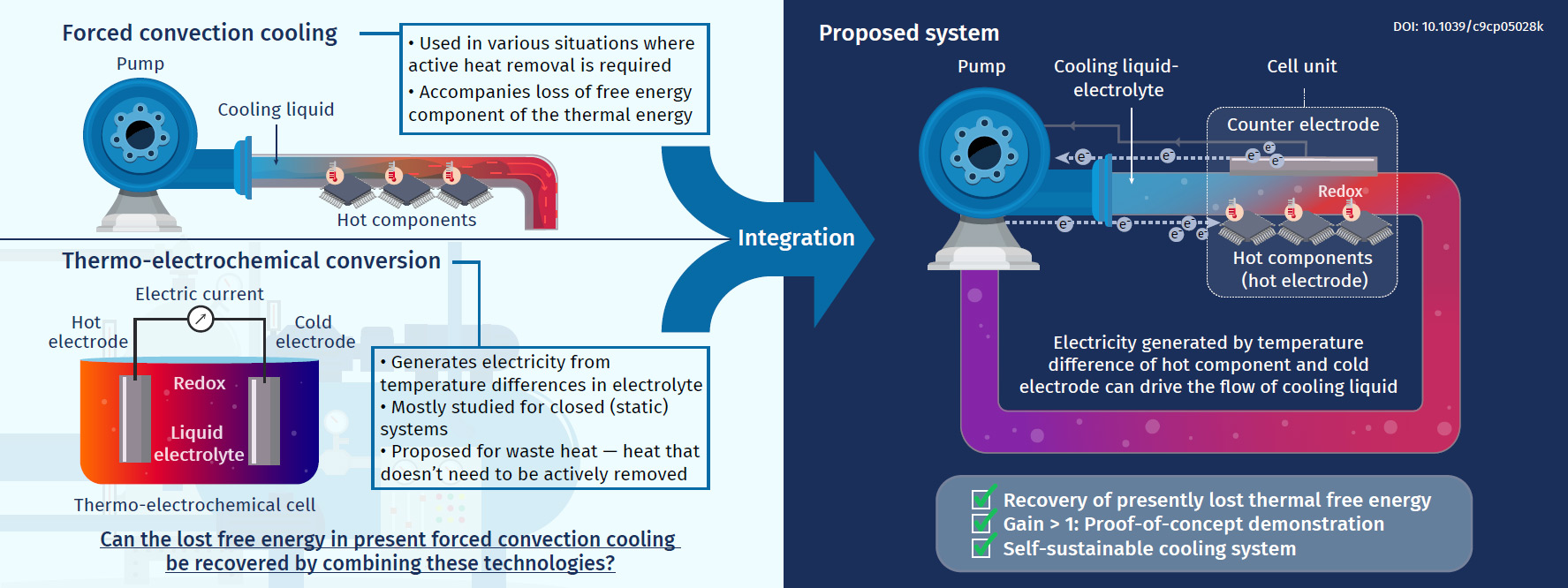
Electrochemical power generation during forced convection cooling to utilize waste heat
In modern civilization, forced convection cooling plays a vital role in a variety of technologies, from microprocessors in data centers, which currently consume 1%–2% of the world's electricity production, to heat engines, including turbines and automobile engines. These technologies require active cooling to avoid thermal failure (for microprocessors) and maximize fuel-to-work conversion efficiency (heat engines). Here, "active cooling" means the prompt removal of a large quantity of thermal energy emitted from a heat source using a working fluid under a large temperature difference. However, such active cooling promptly decreases the exergy of thermal energy, which is the free-energy component of the thermal energy. If this current problem can be resolved, it could have a major impact on the use of thermal energy, which is currently emitted in large quantities but has not received much attention.
The purpose of this research project is to partly recover presently lost exergy in such cooling situations. In particular, we aim at an integration of thermoelectrochemical conversion, which has mostly been studied for stationary conditions using a liquid electrolyte in a closed cell, into forced convection cooling using an electrolyte as the coolant. To fulfill this purpose, we have designed a test cell, in which an electrolyte liquid is forced through a channel formed between two parallel electrodes, and the hot-side electrode simulates an object that needs to be cooled.
So far, we have conducted investigations using ionic liquids (room-temperature molten salts composed entirely of ions) as the solvent of the redox couple; this solution is forced through the cell as a coolant. The virtual non-flammability and non-volatility of ionic liquids are suitable for wide-ranging heat sources where safety is concerned and situations including uses in space where the environment is a vacuum, respectively. From our experiments and computational simulations, several findings regarding the fundamental properties of this kind of forced-flow thermocell have been acquired.
However, to bring this concept to real application, further improvements are required. The required improvements include the design optimization of the geometry of the liquid flowing channel in the cell, the establishment of strategies for scale-up of the cell dimensions, and the optimization of both electrode materials and redox couple chemicals used in the cell.

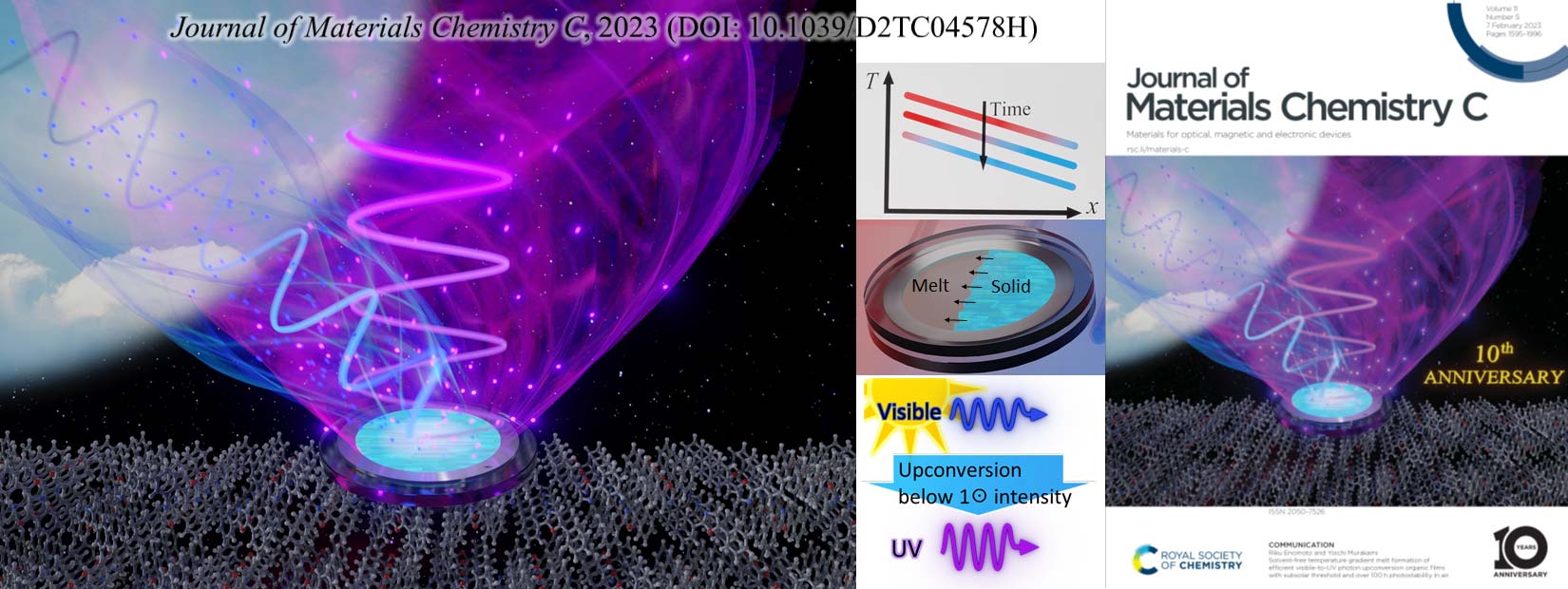
Photon upconversion to increase solar energy utilization efficiency
Light is an electromagnetic wave, as shown by the fact that monochromatic light obtained using a glass prism has a unique wavelength. Light also behaves as a collection of elementary particles called photons, whose existence is manifested by the photoelectric effect. Light energy (photoenergy) is especially important among many possible forms of energy. Sunlight is the only energy supplied to the Earth from space. In addition, light has many important practical functions to convert energy and matter to more useful forms. For example, using photovoltaics, photoenergy can be converted to more useful electrical energy. Using photosynthesis materials or photocatalysts, photoenergy can be converted to various storable forms of chemical energy like sugars, proteins, hydrocarbon fuels, and hydrogen gas.
However, the efficiencies of these photoenergy conversion systems suffer from a fundamental limitation. This originates from the fact that only photons possessing higher energies than the bandgap energy of semiconductors or the gap energy between the highest occupied and lowest unoccupied molecular orbitals of molecular materials can be used. Photons with lower energy than these intrinsic gap energies of materials are wasted at present, regardless of the power of the incident light. Because the energy of a photon is inversely proportional to the wavelength of light, there is a threshold wavelength, which represents a boundary separating the usable and unusable spectral regions of sunlight. That is, incident light that has a longer wavelength than this threshold wavelength is useless for photoenergy conversion, resulting in the aforementioned limitation of conversion efficiency. For example, the water-splitting reaction to generate hydrogen gas by a photocatalyst can usually be achieved only by light that has a wavelength shorter than blue (∼450 nm). Photosynthesis in plants can only be driven by light whose wavelength is red or shorter. Light that can generate electric power in amorphous silicon photovoltaics is limited to wavelengths shorter than dark red (∼720 nm). Therefore, given the solar spectrum on Earth, numerous photoenergy conversion systems display fundamental losses of energy.
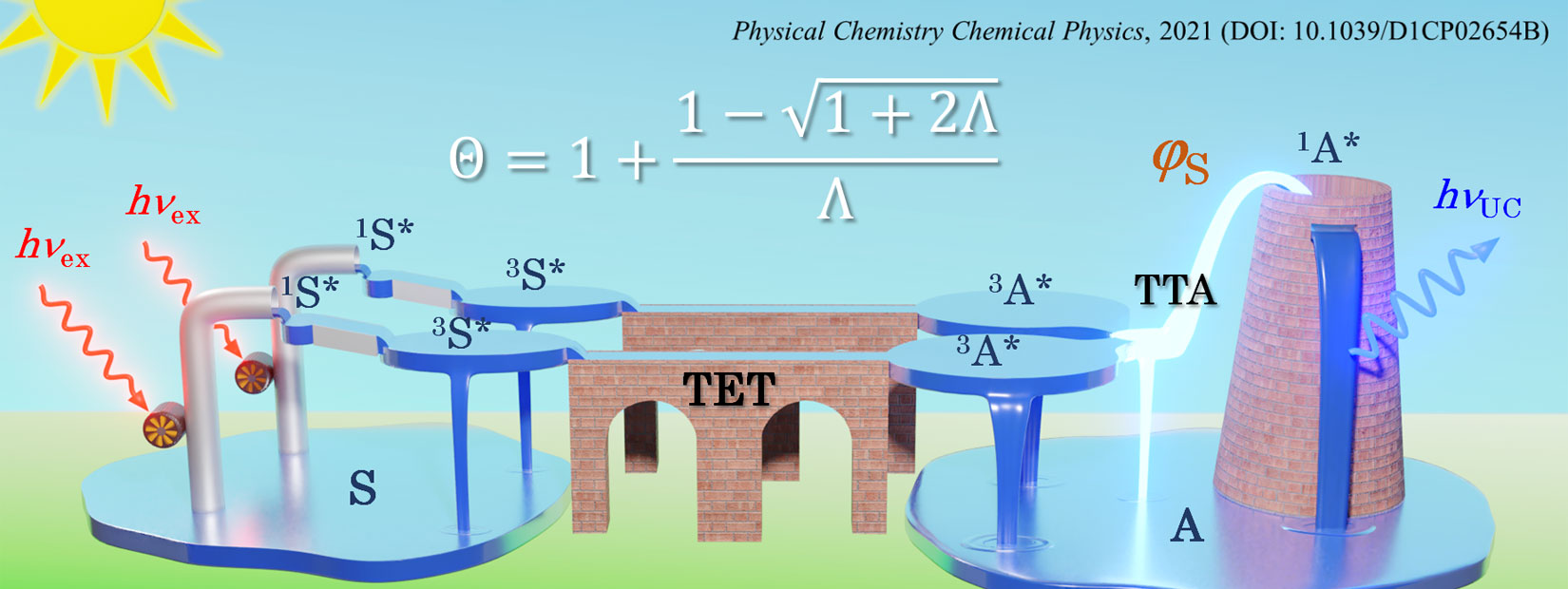
Photon upconversion (UC) is a technology that circumvents these fundamental losses and converts presently unused photons to usable ones. Previously, UC was only possible for high-intensity laser light possessing linear polarization. Recently, a method of UC that uses properly designed intermolecular energy transfer between organic molecules emerged and has been found to be applicable to low-intensity and randomly polarized light, including normal sunlight. Thus, this UC method has great versatility with broader application prospects. Realization of such UC with high efficiency would not only increase the efficiencies of the aforementioned photoenergy conversions but also broaden the possible applications of light.
We started studies on this UC technology soon after its emergence and have reported several unique achievements. This research field is currently rapidly growing, through which exciting frontiers and questions are emerging. Our present aims are to elucidate such questions and develop novel UC materials suitable for application. To attain these goals, we are conducting highly interdisciplinary research encompassing physical chemistry, mechanical engineering, photochemistry, and materials science.