Power Generation from
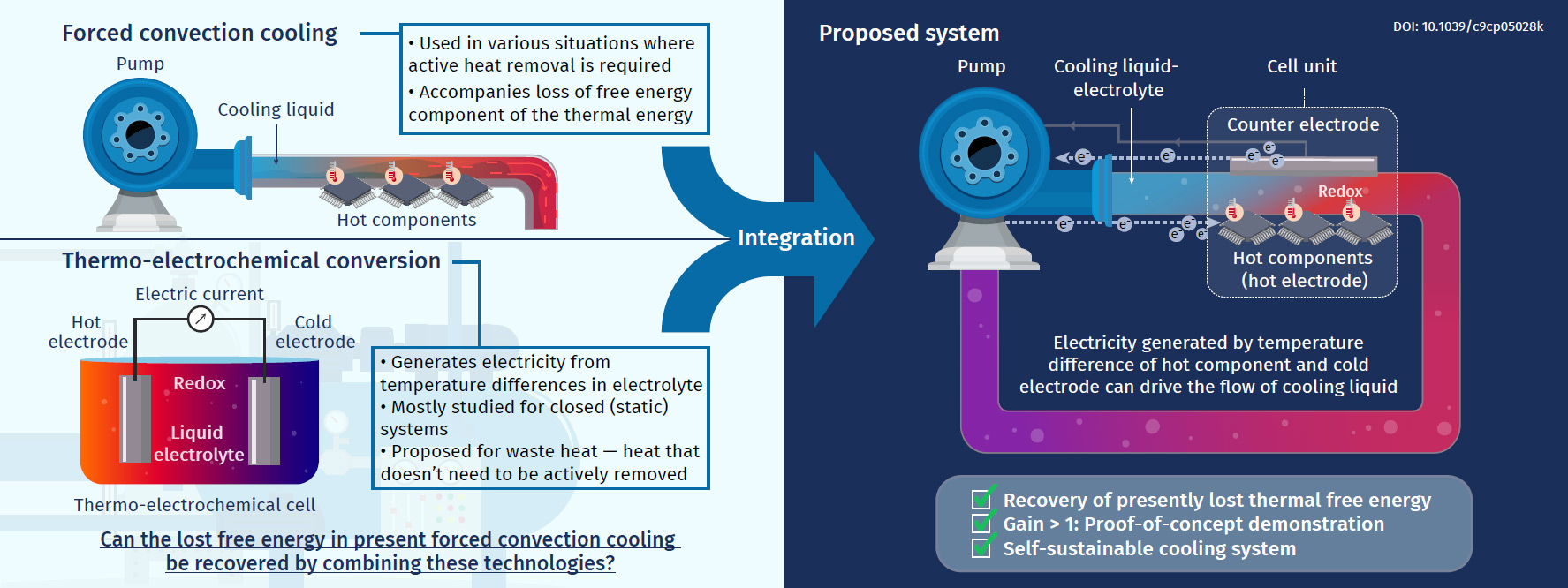
In modern civilization, forced convection cooling plays a vital role in a variety of technologies, from microprocessors in data centers, which currently consume 1%–2% of the world's electricity production, to heat engines, including turbines and automobile engines. These technologies require active cooling to avoid thermal failure (for microprocessors) and maximize fuel-to-work conversion efficiency (heat engines). Here, "active cooling" means the prompt removal of a large quantity of thermal energy emitted from a heat source using a working fluid under a large temperature difference. However, such active cooling promptly decreases the exergy of thermal energy, which is the free-energy component of the thermal energy. If this current problem can be resolved, it could have a major impact on the use of thermal energy, which is currently emitted in large quantities but has not received much attention.
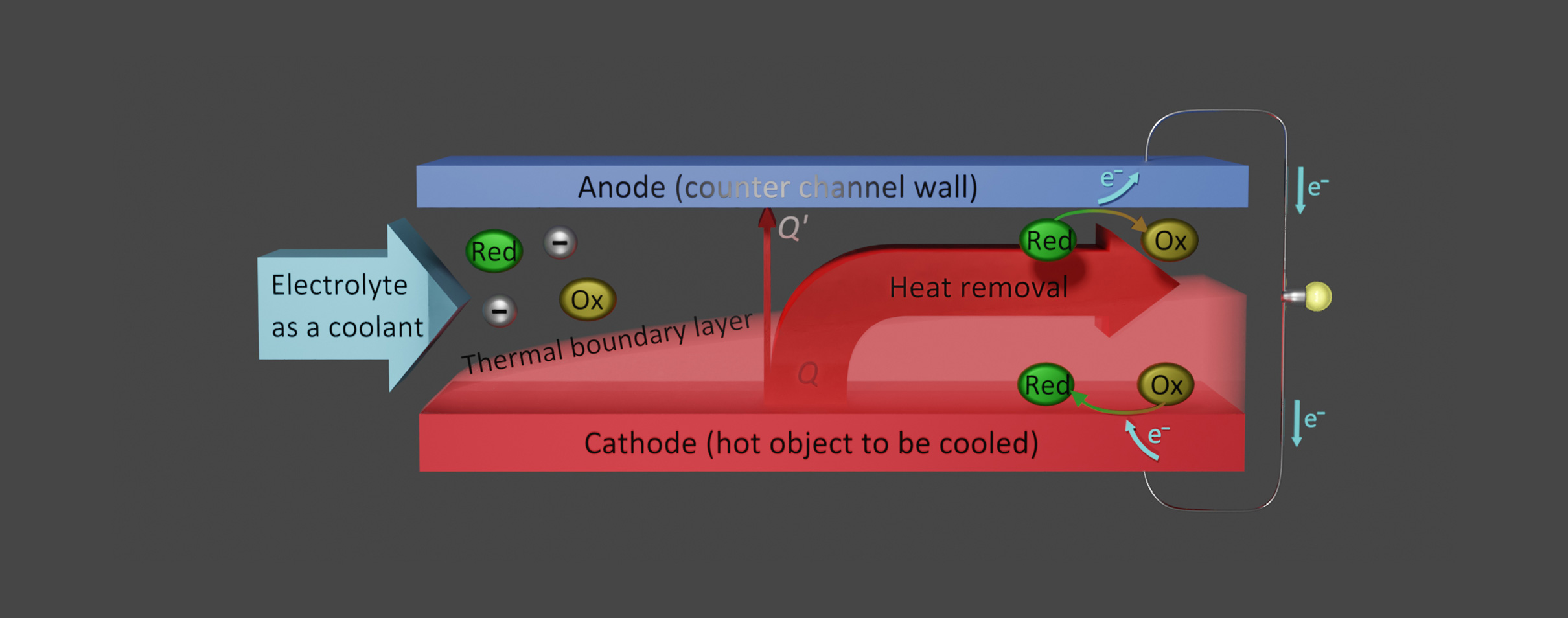
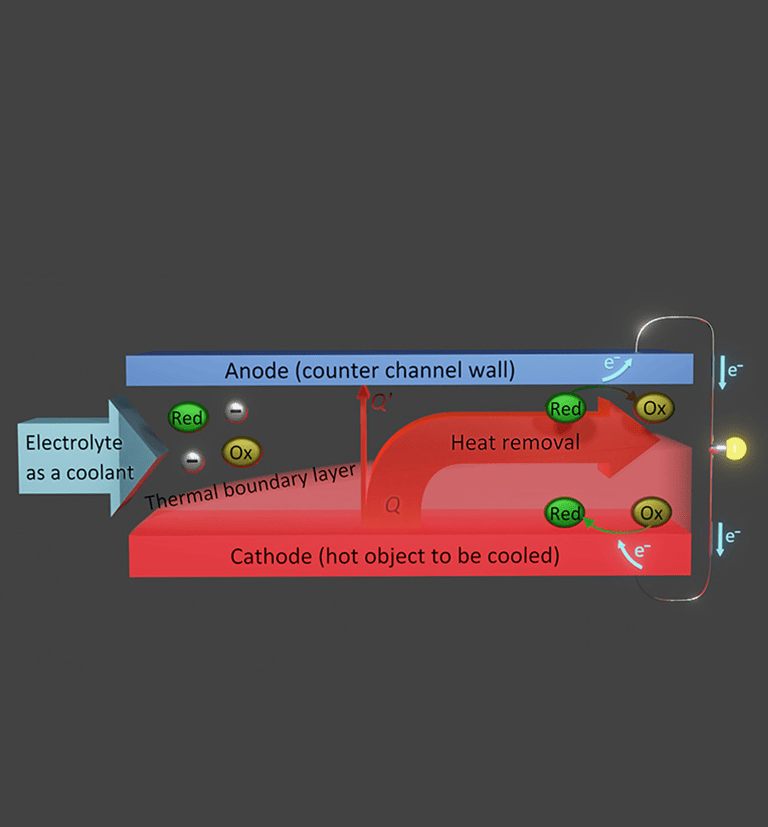
The purpose of this research project is to partly recover presently lost exergy in such cooling situations. In particular, we aim at an integration of thermoelectrochemical conversion, which has mostly been studied for stationary conditions using a liquid electrolyte in a closed cell, into forced convection cooling using an electrolyte as the coolant. To fulfill this purpose, we have designed a test cell, in which an electrolyte liquid is forced through a channel formed between two parallel electrodes, and the hot-side electrode simulates an object that needs to be cooled.
So far, we have conducted investigations using ionic liquids (room-temperature molten salts composed entirely of ions) as the solvent of the redox couple; this solution is forced through the cell as a coolant. The virtual non-flammability and non-volatility of ionic liquids are suitable for wide-ranging heat sources where safety is concerned and situations including uses in space where the environment is a vacuum, respectively. From our experiments and computational simulations, several findings regarding the fundamental properties of this kind of forced-flow thermocell have been acquired.
However, to bring this concept to real application, further improvements are required. The required improvements include the design optimization of the geometry of the liquid flowing channel in the cell, the establishment of strategies for scale-up of the cell dimensions, and the optimization of both electrode materials and redox couple chemicals used in the cell.
Related paper: Development of redox flow thermoelectric power generation that achieves both cooling and power generation (Electrochemistry, 2024)